 Figure 1.
The black smoke at the Twin Towers was indicative of the incomplete
combustion usually associated with low-temperature fires. Office fires
cannot melt steel, even given optimal conditions.A December 2001 paper, "Why Did the World Trade Center Collapse? Science, Engineering, and Speculation,"
dismissed early reports about molten steel at the demolished World
Trade Center. Dr. Thomas W. Eagar, a professor of materials engineering
and engineering systems at the Massachusetts Institute of Technology,
and his graduate research student, Christopher Musso, pointed out that
the theoretical maximum temperature of a building fire (maximum
1000°C/1800°F) is not even close to the melting point of steel
(approximately 1500°C/2750°F). And they noted that the observed black
smoke emanating from the Twin Towers was consistent with a typical
oxygen-starved building fire. Figure 1.
The black smoke at the Twin Towers was indicative of the incomplete
combustion usually associated with low-temperature fires. Office fires
cannot melt steel, even given optimal conditions.A December 2001 paper, "Why Did the World Trade Center Collapse? Science, Engineering, and Speculation,"
dismissed early reports about molten steel at the demolished World
Trade Center. Dr. Thomas W. Eagar, a professor of materials engineering
and engineering systems at the Massachusetts Institute of Technology,
and his graduate research student, Christopher Musso, pointed out that
the theoretical maximum temperature of a building fire (maximum
1000°C/1800°F) is not even close to the melting point of steel
(approximately 1500°C/2750°F). And they noted that the observed black
smoke emanating from the Twin Towers was consistent with a typical
oxygen-starved building fire.
Eagar and Musso concluded that the actual temperature most likely
remained below 650°C/1200°F. In so doing, they dispelled the myth that
the jet fuel could have made the fires unusually hot, noting that it was
"highly unlikely" that the temperature rose above 800°C/1470°F.
AE911Truth agrees that the jet-fuel-induced fires in the Twin Towers could not have melted steel.
But because more recent reports confirm the presence of molten steel and molten iron both
during and after the 9/11 event, it must be determined what actually
melted those two metals and in so doing demolished two of the world's
tallest steel-frame skyscrapers.
The Official Fired-Based Hypothesis Cannot Account for the Stream of Liquid Metal Pouring Out of the South Tower
Figure 2.
Yellow-white glowing molten metal is seen pouring from the South Tower
just minutes before its collapse. Accompanying white smoke was sometimes
visible. NIST did not investigate the phenomenon. http://youtu.be/OmuzyWC60eE
T Figure 3.
A thermite reaction generates yellow-white hot molten iron at well over
2500°C/4000°F and white smoke. This type of material can melt and cut
steel beams.he National Institute of Standards and Technology (NIST) did document the flow of molten metal
pouring out of the South Tower during the final seven minutes before
its collapse, noting the accompanying "unusual bright flame" and "plume
of white smoke." However, NIST failed to investigate the phenomenon,
dismissing it as molten aluminum from the crashed jet, which melts at
only 660°C/1220°F.
NIST's hypothesis may seem plausible at first. But Dr. Steven Jones demonstrates in his 2006 paper "Why Indeed Did the WTC Buildings Completely Collapse?" that the official government hypothesis is untested and implausible.
Dr. Jones' paper reveals that the initial bright yellow-white glow of
the expelled liquid was consistent with a glowing stream of molten iron
from "a nearby thermite reaction zone," and the expected white smoke
(aluminum oxide off-gassing) supports that conclusion. NIST must rely on
its claim of molten aluminum in order to validate its official
fire-based explanation, because office fires cannot generate the extreme
temperature required to melt steel or iron. The fundamental flaw of the
aluminum hypothesis, though, is that the implied temperature of the
white glow remains above 1200°C/2200°F, regardless of the metal involved. An independent researcher suggested that the molten substance could be lead from storage batteries,
but this explanation fails — as do all hypotheses based on alternative
metals — because the temperature required for the yellow-white glow of
the metal is beyond the capability of the building fire. Figure 3.
A thermite reaction generates yellow-white hot molten iron at well over
2500°C/4000°F and white smoke. This type of material can melt and cut
steel beams.he National Institute of Standards and Technology (NIST) did document the flow of molten metal
pouring out of the South Tower during the final seven minutes before
its collapse, noting the accompanying "unusual bright flame" and "plume
of white smoke." However, NIST failed to investigate the phenomenon,
dismissing it as molten aluminum from the crashed jet, which melts at
only 660°C/1220°F.
NIST's hypothesis may seem plausible at first. But Dr. Steven Jones demonstrates in his 2006 paper "Why Indeed Did the WTC Buildings Completely Collapse?" that the official government hypothesis is untested and implausible.
Dr. Jones' paper reveals that the initial bright yellow-white glow of
the expelled liquid was consistent with a glowing stream of molten iron
from "a nearby thermite reaction zone," and the expected white smoke
(aluminum oxide off-gassing) supports that conclusion. NIST must rely on
its claim of molten aluminum in order to validate its official
fire-based explanation, because office fires cannot generate the extreme
temperature required to melt steel or iron. The fundamental flaw of the
aluminum hypothesis, though, is that the implied temperature of the
white glow remains above 1200°C/2200°F, regardless of the metal involved. An independent researcher suggested that the molten substance could be lead from storage batteries,
but this explanation fails — as do all hypotheses based on alternative
metals — because the temperature required for the yellow-white glow of
the metal is beyond the capability of the building fire.
 Figure 4.
Molten aluminum appears silvery when poured in daylight conditions,
even if initially heated to the yellow-white temperature range in the
crucible.Dr. Jones also notes that molten aluminum appears
silvery as it melts at 660°C/1220°F, and that it remains silvery when
poured in daylight conditions, regardless of the temperature. It is
theoretically possible to continue heating liquid aluminum way past its
melting point and into the yellow-white temperature range, but the
office fire was not a plausible source for such high temperatures, and
there was no crucible to contain liquid aluminum for continued heating.
Put another way, even if the building fire could have somehow provided
the needed temperature for the yellow-white glow, the unrestrained
aluminum would have melted and trickled away before it could achieve
such a temperature. This problem also rules out other proposed
alternative metals — lead, for example — which have similarly low
melting points.
Finally, Dr. Jones adds that even if liquid aluminum could have been
restrained long enough to make it glow white, it would still have
appeared silvery within the first two meters of falling through the air
in daylight conditions, due to its high reflectivity and low emissivity. Figure 4.
Molten aluminum appears silvery when poured in daylight conditions,
even if initially heated to the yellow-white temperature range in the
crucible.Dr. Jones also notes that molten aluminum appears
silvery as it melts at 660°C/1220°F, and that it remains silvery when
poured in daylight conditions, regardless of the temperature. It is
theoretically possible to continue heating liquid aluminum way past its
melting point and into the yellow-white temperature range, but the
office fire was not a plausible source for such high temperatures, and
there was no crucible to contain liquid aluminum for continued heating.
Put another way, even if the building fire could have somehow provided
the needed temperature for the yellow-white glow, the unrestrained
aluminum would have melted and trickled away before it could achieve
such a temperature. This problem also rules out other proposed
alternative metals — lead, for example — which have similarly low
melting points.
Finally, Dr. Jones adds that even if liquid aluminum could have been
restrained long enough to make it glow white, it would still have
appeared silvery within the first two meters of falling through the air
in daylight conditions, due to its high reflectivity and low emissivity.
 Figure 5.
The liquid metal cannot be aluminum, for it remains orange-yellow,
despite falling several hundred feet in broad daylight. NIST states that
aluminum "can display an orange glow" if blended with organic
materials, but Dr. Jones has experimentally invalidated this theory by
demonstrating that organics and molten aluminum do not mix. Thus,
the liquid metal seen pouring out of the South Tower could not have
been aluminum, since it remains yellow in broad daylight, despite
falling several hundred feet through the air.
NIST tries to circumvent this problem with the untested proposition
that the observed glow could be due to the mixing of aluminum with
combustible organic materials from the building's interior. But Dr. Jones has actually performed the experiments
that soundly refute NIST's hypothesis. As he puts it, "This is a key to
understanding why the aluminum does not 'glow orange' due to
partially-burned organics 'mixed' in (per NIST theory), because they do not
mix in! My colleague noted that, just like oil and water, organics and
molten aluminum do not mix. The hydrocarbons float to the top, and there
burn — and embers glow, yes, but just in spots. The organics clearly do
not impart to the hot liquid aluminum an 'orange glow' when it falls, when you actually do the experiment!" Figure 5.
The liquid metal cannot be aluminum, for it remains orange-yellow,
despite falling several hundred feet in broad daylight. NIST states that
aluminum "can display an orange glow" if blended with organic
materials, but Dr. Jones has experimentally invalidated this theory by
demonstrating that organics and molten aluminum do not mix. Thus,
the liquid metal seen pouring out of the South Tower could not have
been aluminum, since it remains yellow in broad daylight, despite
falling several hundred feet through the air.
NIST tries to circumvent this problem with the untested proposition
that the observed glow could be due to the mixing of aluminum with
combustible organic materials from the building's interior. But Dr. Jones has actually performed the experiments
that soundly refute NIST's hypothesis. As he puts it, "This is a key to
understanding why the aluminum does not 'glow orange' due to
partially-burned organics 'mixed' in (per NIST theory), because they do not
mix in! My colleague noted that, just like oil and water, organics and
molten aluminum do not mix. The hydrocarbons float to the top, and there
burn — and embers glow, yes, but just in spots. The organics clearly do
not impart to the hot liquid aluminum an 'orange glow' when it falls, when you actually do the experiment!"
 Figure 6.
Several reports document the abundant iron-rich spheres in the WTC
dust, confirming the formation of molten iron "during the event,"
according to an independent study of the South Tower dust by the RJ Lee
Group.Dr. Jones et al confirmed the finding of molten iron in a 2008 paper, "Extremely high temperatures during the World Trade Center destruction," which documents their discovery of iron-rich microspheres in WTC dust samples from two independent sources. Figure 6.
Several reports document the abundant iron-rich spheres in the WTC
dust, confirming the formation of molten iron "during the event,"
according to an independent study of the South Tower dust by the RJ Lee
Group.Dr. Jones et al confirmed the finding of molten iron in a 2008 paper, "Extremely high temperatures during the World Trade Center destruction," which documents their discovery of iron-rich microspheres in WTC dust samples from two independent sources.
The Official Fire-Based Hypothesis Cannot Account for the
Red-Hot Steel Beams and Pools of Molten Metal Seen During the First
Weeks of Clean-up
Numerous professionals
have testified that they saw "molten steel" beneath the Ground Zero
rubble. But they are not metallurgists, so how did they know enough to
have identified it correctly as steel?
NIST dodges the answer to that question by claiming that there was no
molten metal to investigate. NIST engineer John Gross, co-project
leader of the official investigation, denied the existence of the witness reports.
So we must look to the context, which provides a clear answer: The
primary structural components of the WTC Towers were steel columns,
steel beams, and steel floor trusses. Thus, steel was the only option that the witnesses had when they identified the unmistakable structural steel components coming out molten from under the rubble. Specific statements
from these witnesses about "molten steel beams" and beams "dripping
molten steel" dispel any remaining doubts. The reported pools of molten
metal under the rubble must also have contained some of that molten
steel, and perhaps molten iron from thermitic cutting charges as well.
 Figure 7.
An excavator picks up metal rubble from deep within the pile, and some
of it is dripping a yellow-white hot liquid metal at or above
1200°C/2200°F. This is approximately double the temperature that can be
reasonably expected from an oxygen-starved fire.Dr. Jones addressed the evidence from yet another angle, pointing out that "we can rule out some metals based on available data."
A photograph taken 16 days after the 9/11 event shows an excavator
grabbing debris that remains solid even though it is glowing in the
salmon-to-yellow hot range. Dr. Jones notes that the solid
metal, glowing in the 845°C/1550°F to 1080°C/1975°F temperature range,
could not have been aluminum, lead, or other metals with low melting
points, because none of them could have remained solid in this range.
The glowing debris was also dripping liquid metal that appears to
have a bright yellow-white glow, which leads to the conclusion that the
maximum temperature of the glowing rubble was probably above
1200°C/2200°F — consistent with the yellow-white hot glow of molten
steel in a foundry.
What makes this so remarkable is that anything over 1000°C/1800°F is
above the maximum temperature of a perfectly ventilated fire, and is
therefore way beyond the temperature limit of an oxygen-starved fire under the rubble.
The liquid metal could not have been aluminum because it would have
had a silvery appearance as it dripped away at its 660°C/1220°F melting
point. And we suspect that the powerful floodlights at the demolition
site would have made it appear silver-colored, anyway, regardless of the
temperature, due to the low emissivity and high reflectivity of
aluminum. Dr. Jones adds that the metal in question also needed a
"fairly low heat conductivity and a relatively large heat capacity" to
remain red hot and even molten for several weeks under the rubble — two
traits that identify the metal as steel or iron. Figure 7.
An excavator picks up metal rubble from deep within the pile, and some
of it is dripping a yellow-white hot liquid metal at or above
1200°C/2200°F. This is approximately double the temperature that can be
reasonably expected from an oxygen-starved fire.Dr. Jones addressed the evidence from yet another angle, pointing out that "we can rule out some metals based on available data."
A photograph taken 16 days after the 9/11 event shows an excavator
grabbing debris that remains solid even though it is glowing in the
salmon-to-yellow hot range. Dr. Jones notes that the solid
metal, glowing in the 845°C/1550°F to 1080°C/1975°F temperature range,
could not have been aluminum, lead, or other metals with low melting
points, because none of them could have remained solid in this range.
The glowing debris was also dripping liquid metal that appears to
have a bright yellow-white glow, which leads to the conclusion that the
maximum temperature of the glowing rubble was probably above
1200°C/2200°F — consistent with the yellow-white hot glow of molten
steel in a foundry.
What makes this so remarkable is that anything over 1000°C/1800°F is
above the maximum temperature of a perfectly ventilated fire, and is
therefore way beyond the temperature limit of an oxygen-starved fire under the rubble.
The liquid metal could not have been aluminum because it would have
had a silvery appearance as it dripped away at its 660°C/1220°F melting
point. And we suspect that the powerful floodlights at the demolition
site would have made it appear silver-colored, anyway, regardless of the
temperature, due to the low emissivity and high reflectivity of
aluminum. Dr. Jones adds that the metal in question also needed a
"fairly low heat conductivity and a relatively large heat capacity" to
remain red hot and even molten for several weeks under the rubble — two
traits that identify the metal as steel or iron.
 Figure 8.
The reddish (rust) color of similar, previously-molten, Ground Zero
debris, shown in this warehouse photo, indicates the presence of iron or
steel.
A New York warehouse (see Figure 8) stores similar, but solidified,
Ground Zero debris, which supports the conclusion that the excavator at
Ground Zero is picking up iron or steel. This solidified lump has the
embedded remains of the steel beams seen all around the excavator. Also
fused to the warehouse lump are steel reinforcing bars that look like
the rods that are seen glowing hot in the claw (see Figure 7). These
embedded remains display the characteristic reddish color of rusted iron
or steel.
The PBS documentary "Relics from the Rubble"
shows a similar lump of fused molten concrete and molten steel, which
became known as "the meteorite." The leader of the Ground Zero artifact
recovery, architect Bart Voorsanger,
describes the object, which must have weighed several tons, as "fused
element[s] of steel ... molten steel and concrete — and all of these
things ... all fused by the heat."
Thermitic Materials Can Account for the Molten Iron and the Molten Steel
Since building fires cannot account for the reported molten steel
beams in the Ground Zero rubble, the official fire-based explanation for
the collapses of the WTC buildings must be false. An independent study
by the RJ Lee Group actually used the previously liquefied iron-rich spheres as a signature marker to distinguish the WTC dust from normal building dust, because they were so abundant.
The official explanation also fails to account for the plenitude of
iron-rich spheres, which happen to be yet another signature marker for a
thermite reaction. Since thermitic materials can actually cut and melt steel beams, evidence of this type of material in the dust provides a plausible explanation for the observed liquid iron and steel: Thermitic cutting charges melt a slit through the steel beams via a directed blast of molten iron, leaving behind the expected residues of molten iron from the charges and molten steel from the beams.
Chemist Kevin Ryan notes that NIST violated the NFPA 921
investigative standard by denying the evidence of molten iron and
molten steel, and by refusing to look for pyrotechnic and explosive
materials. This is especially suspicious, according to Ryan, because
"NIST had considerable connections to nano-thermites, both before and
during the WTC investigation." Figure 8.
The reddish (rust) color of similar, previously-molten, Ground Zero
debris, shown in this warehouse photo, indicates the presence of iron or
steel.
A New York warehouse (see Figure 8) stores similar, but solidified,
Ground Zero debris, which supports the conclusion that the excavator at
Ground Zero is picking up iron or steel. This solidified lump has the
embedded remains of the steel beams seen all around the excavator. Also
fused to the warehouse lump are steel reinforcing bars that look like
the rods that are seen glowing hot in the claw (see Figure 7). These
embedded remains display the characteristic reddish color of rusted iron
or steel.
The PBS documentary "Relics from the Rubble"
shows a similar lump of fused molten concrete and molten steel, which
became known as "the meteorite." The leader of the Ground Zero artifact
recovery, architect Bart Voorsanger,
describes the object, which must have weighed several tons, as "fused
element[s] of steel ... molten steel and concrete — and all of these
things ... all fused by the heat."
Thermitic Materials Can Account for the Molten Iron and the Molten Steel
Since building fires cannot account for the reported molten steel
beams in the Ground Zero rubble, the official fire-based explanation for
the collapses of the WTC buildings must be false. An independent study
by the RJ Lee Group actually used the previously liquefied iron-rich spheres as a signature marker to distinguish the WTC dust from normal building dust, because they were so abundant.
The official explanation also fails to account for the plenitude of
iron-rich spheres, which happen to be yet another signature marker for a
thermite reaction. Since thermitic materials can actually cut and melt steel beams, evidence of this type of material in the dust provides a plausible explanation for the observed liquid iron and steel: Thermitic cutting charges melt a slit through the steel beams via a directed blast of molten iron, leaving behind the expected residues of molten iron from the charges and molten steel from the beams.
Chemist Kevin Ryan notes that NIST violated the NFPA 921
investigative standard by denying the evidence of molten iron and
molten steel, and by refusing to look for pyrotechnic and explosive
materials. This is especially suspicious, according to Ryan, because
"NIST had considerable connections to nano-thermites, both before and
during the WTC investigation."
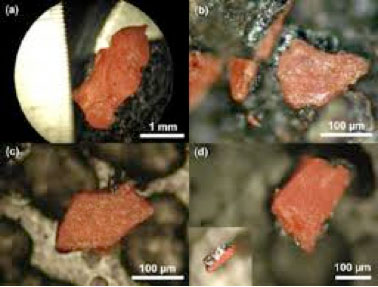 Figure 9.
Dr. Niels Harrit leads an international team of scientists that
documents the finding of red-gray nano-thermite chips in four
independently-collected WTC dust samples. This material ignites and
forms the iron-rich spheres that were so abundant in the dust.Although
NIST has failed to fulfill its duty, a team of nine scientists has
investigated samples of dust from the collapsed Twin Towers and has
documented the discovery of microscopic-but-intact remnants of
nano-thermite. This type of energetic material can be easily tailored to
be either pyrotechnic or explosive.
Chemist Dr. Niels Harrit leads the team of scientists, which includes
Dr. Steven Jones and Kevin Ryan. Their investigation resulted in the
2009 peer-reviewed paper, "Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe." Harrit et al identify only one of
the thermitic materials that must have been used, but they do not
attempt to ascertain if the cutting charges were composed of this
particular material. Chemical engineer Mark Basile has already independently verified the conclusion of their paper. His study is still being completed and will hopefully be published by the end of 2014.
Kevin Ryan summarizes the molten metal evidence that we have reviewed
here, as well as additional evidence in favor of thermitic materials,
in his December 2013 article, "9/11 Truth: How to Debunk WTC Thermite at Ground Zero."
Ryan concludes that the evidence is "extensive and compelling," and
that the suspected controlled demolition of the WTC buildings via
thermitic materials is now "a tested and proven theory." And, as
demonstrated above, thermite remains the only viable theory that
provides a logical explanation for the liquefied iron and steel found in
the World Trade Center rubble. Figure 9.
Dr. Niels Harrit leads an international team of scientists that
documents the finding of red-gray nano-thermite chips in four
independently-collected WTC dust samples. This material ignites and
forms the iron-rich spheres that were so abundant in the dust.Although
NIST has failed to fulfill its duty, a team of nine scientists has
investigated samples of dust from the collapsed Twin Towers and has
documented the discovery of microscopic-but-intact remnants of
nano-thermite. This type of energetic material can be easily tailored to
be either pyrotechnic or explosive.
Chemist Dr. Niels Harrit leads the team of scientists, which includes
Dr. Steven Jones and Kevin Ryan. Their investigation resulted in the
2009 peer-reviewed paper, "Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe." Harrit et al identify only one of
the thermitic materials that must have been used, but they do not
attempt to ascertain if the cutting charges were composed of this
particular material. Chemical engineer Mark Basile has already independently verified the conclusion of their paper. His study is still being completed and will hopefully be published by the end of 2014.
Kevin Ryan summarizes the molten metal evidence that we have reviewed
here, as well as additional evidence in favor of thermitic materials,
in his December 2013 article, "9/11 Truth: How to Debunk WTC Thermite at Ground Zero."
Ryan concludes that the evidence is "extensive and compelling," and
that the suspected controlled demolition of the WTC buildings via
thermitic materials is now "a tested and proven theory." And, as
demonstrated above, thermite remains the only viable theory that
provides a logical explanation for the liquefied iron and steel found in
the World Trade Center rubble.
|


















































